qordata hosted an interactive webinar with Sarah DiFransesca from Dinsmore & Shohl LLP and Leslie Restanio from Amneal Pharmaceuticals for analyzing the recently rolled out OIG’s Special Fraud Alert for Speaker Programs.
The Special Fraud Alert highlights the fraud and abuse risks associated with the offer, payment, solicitation, or receipt of remuneration relating to speaker programs by life sciences companies. It raises questions on the educational value of speaker programs, and stated programs are thus subject to scrutiny.
Why the need for this Special Fraud Alert arise?
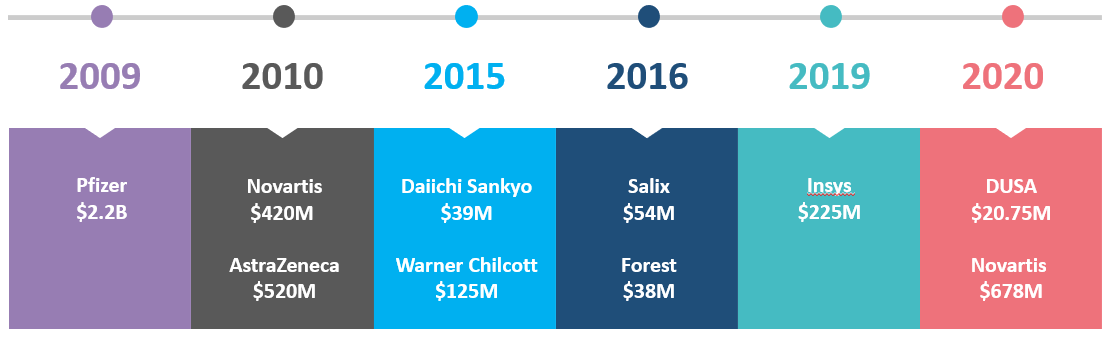
Numerous False Claims Act (FCA) settlements with OIG and DOJ involved allegations that pharmaceutical and medical device companies violated the Anti-Kickback Statute (AKS) by paying remuneration to HCPs for speaker programs with the intent of induce prescriptions of company products.
The OIG, therefore, considers that one purpose of the remuneration to the HCP speaker and attendees is to induce or reward referrals which is a violation of the AKS.
In order to ensure transparency, the OIG Special Fraud Alert addresses the issues that can possibly make life sciences companies come under their watch for violating Anti-Kickback Statute.
Fraud Alert makes it clear that risk relates to all forms of remuneration provided in connection with speaker programs, such as:
- HCP speaker remuneration
- Meals/alcohol provided to HCP attendees
- Speaker training
For more information on OIG Special Fraud Alert, the speaker program characteristics that could potentially be suspect under the AKS listed in this alert and how as a compliance professional, you can be mindful about it, watch our webinar “Analyzing the OIG Special Fraud Alert” here.